“We can bring any computer to its knees”
“We can bring any computer to its knees"
Markus Oppel is a Senior Scientist at the University of Vienna. He explains how theoretical chemistry can bring even the most powerful supercomputer to its knees, why quantum computers exist in the first place, and how a single sulphur atom can increase the risk of skin cancer in transplant patients. A conversation about chance, computing power, and teamwork.
Bettina Benesch
Dr Oppel, you work as a Senior Scientist in Theoretical Chemistry at the University of Vienna. Was there a key moment in your life when you thought: “Theoretical chemistry forever”?
Markus Oppel: I had that key moment in my third semester studying chemistry, when we started physical chemistry, which is closely related to theoretical chemistry. Quantum mechanics, the Schrödinger equation – all of it fascinated me, and I knew: this is the field I want to stay in.
Today you also work in high-performance and quantum computing. How did that come about?
Markus Oppel: I’d been programming since I was a teenager. At 18, though, I didn’t realise you could actually study something like that.

Until I took my first programming courses at university and realised I could combine my enthusiasm for computers with chemistry. Today, I’m partly responsible for administering our in-house computing cluster, supporting our researchers with their projects, and advising them on which supercomputer is best suited for their calculations.
Supercomputers and quantum computers have always been linked to chemistry, haven’t they?
Markus Oppel: Exactly. In 1926, Schrödinger published his famous papers containing the equation that mathematically describes quantum mechanics. Not long afterwards, people began thinking about how to apply it to chemical problems. At that time, of course, it was all pencil-and-paper work – you couldn’t actually use a computer. But in the 1950s, the first computers appeared, and quantum chemists were among the first to use them, because you can’t solve thousands of integrals by hand if you want to calculate the structure of a molecule.
“
So theoretical chemistry became the guinea pig of computing in general and of high-performance computing in particular, because we can systematically refine and complicate every calculation – which, of course, always demands more computing power.
„
So theoretical chemistry became a sort of guinea pig for computing in general – and for high-performance computing in particular – because we can make every calculation more complex and more precise, which naturally requires ever more computing power. You could say: if we want to, we can bring any computer to its knees.
Let’s stay with quantum computing for a moment. It feels like one of those hot topics everyone talks about, but few people really understand. How do you see it?
Markus Oppel: That’s true – everyone has their own opinion about it.
Can you explain quantum computing in a way that a layperson can understand?
Markus Oppel: There’s this famous quote from Richard Feynman, who won the Nobel Prize in Physics in 1965 along with two other researchers. He supposedly said – perhaps casually over a beer or a glass of wine – something along the lines of: when we describe quantum systems and calculate with them, we use classical methods on classical computers. Instead, we should use quantum-based methods to simulate quantum systems.
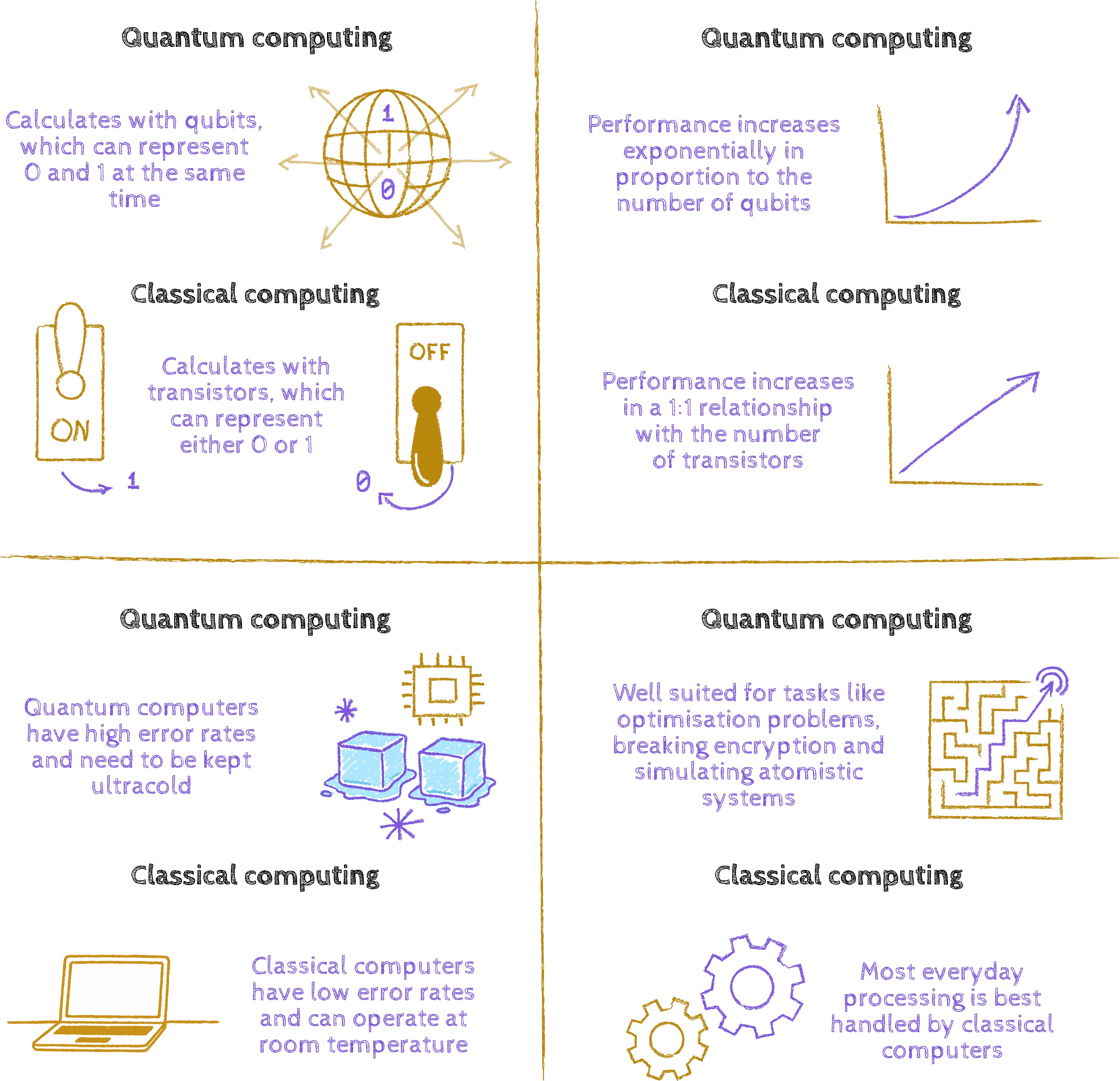
What exactly did he mean by that?
Markus Oppel: There’s a fundamental difference between quantum mechanics and classical mechanics. In a classical computer, everything is either zero or one. Whether it’s a smartphone or a supercomputer, it’s all about zeros and ones being connected or moved around. But quantum mechanics isn’t just zeros and ones – there are those famous in-between states, the superpositions.
Feynman had the crazy – or rather obvious – idea that if we need to describe systems that are zeros, ones, and everything in between, then we need a mechanical and technical system that can actually do that. Not an ordinary computer, which can only process zeros and ones. Today’s quantum computers don’t use transistors, circuits, and classical electronics to represent zeros or ones. They use quantum systems of various kinds that can represent those mixed states where several possibilities exist simultaneously.
“
The know-how is there, but when it comes to quantum computers, we are currently at the technical level where today’s computers were back in the 1930s.
„
Where are we now, technologically speaking, with quantum computers?
Markus Oppel: With classical computers we measure performance in bits; with quantum computers, in qubits. The quantum computers we have today have at most a few thousand qubits. But for a quantum algorithm to truly work and outperform a normal computer, you’d need tens or hundreds of thousands of qubits. That’s the main issue: we know how it works in principle, but technically we’re still where classical computers were in the 1930s.
So we know how it works in theory, but it’s not yet practical?
Markus Oppel: Exactly. And the big question is: when will it happen? It will, of course, progress faster than classical computing did. I’d say, optimistically, that students starting university today will probably run their simulations on quantum computers in 20 or 30 years.
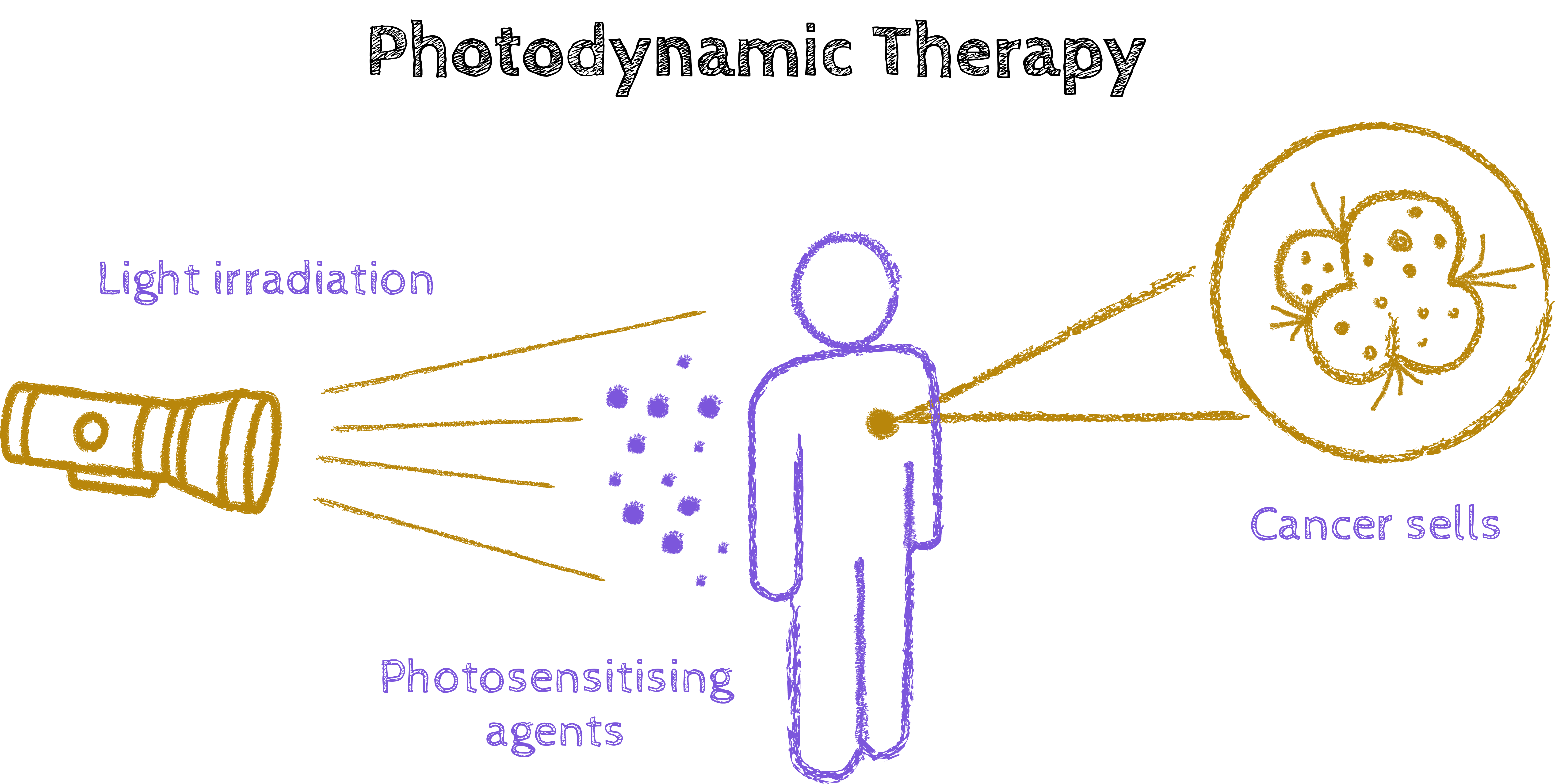
In the meantime, we make do with high-performance computing. You support researchers with their simulations on supercomputers around the world. What have been the most exciting projects in recent years?
Markus Oppel: In our research group, everything revolves around light – on a fundamental level. We’re interested in what a molecule does after it’s been excited by light. Some time ago, we worked on light-activated platinum complexes that could be used in cancer therapy, using the Austrian supercomputer VSC.
We combined two fascinating ideas: since the 1970s, there’s been a platinum compound approved for cancer therapy. Separately, there’s a treatment known as photodynamic therapy, where a drug remains inactive in tumour tissue until the tumour is illuminated. Only then does the light activate the drug’s therapeutic effect, which in turn attacks the cancer cells. Our idea was to take the concept of these platinum compounds – which already work as anti-tumour agents – and modify them so that they only become active when exposed to light.
That hadn’t been done before?
Markus Oppel: Not with platinum, as far as I know. There’s plenty of research, but the drugs approved for photodynamic therapy come from entirely different classes of compounds.
What exactly did you study in this project?
Markus Oppel: In our project, we started with a platinum atom bonded to various other molecules. When exposed to light, these highly reactive fragments detach, which can then destroy the tumour cell. We wanted to understand precisely how this detachment works and what type of light is needed to trigger it. In general, red light is preferred over blue light in photodynamic therapy. Red light penetrates deeper into tissue, which means it can also be used to irradiate tumours located deeper beneath the skin.
“
In our project, we start with a platinum atom to which various other molecules are attached. When exposed to light, these fragments are released – and they can destroy the tumour cell.
„
What role does your research play in the overall drug development process?
Markus Oppel: Drug design typically takes more than ten years, and our work is right at the beginning. But even before that, it’s about understanding the disease itself. You first define what actually causes a particular illness – often some protein in the body that doesn’t function properly. The next question is: can we cure or control the disease by blocking that protein or enzyme? And how? So it’s a kind of screening process, where you test countless possible compounds to see whether one or more interact with that enzyme.
Nowadays, that’s done first on computers. The big advantage is that software can test thousands of compounds and even suggest new molecules we wouldn’t have thought of ourselves. After such a simulation, you might end up with a hundred promising candidates out of 20,000 molecules. Those then go into the lab for testing.
But that’s only the first half: once you have a single compound that passes the lab tests, you still have to find out whether you can actually produce it, and whether it can be given to a patient safely. In short, drug design is a long process, and we’re right at the start of it. In our group, it’s always about how molecules interact with other substances – especially with light.
“
Every chemist knows: most organic substances break down fairly quickly when exposed to sunlight. So the question arises – why, and how, are humans, as well as plants and animals, able to survive even very intense sunlight?
„
How do you study that in practice?
Markus Oppel: We have our own simulation techniques, including software we’ve developed over the past ten years. This allows us to study, for example, why our cells don’t die when exposed to sunlight. Every chemist knows that most organic substances degrade quickly in sunlight. So the question is: how do humans – and plants and animals – manage to survive even intense sunlight? There are many reasons, and we’re studying them.
For instance, we looked at two of the DNA bases: adenine and guanine. In computer simulations, we could show what happens to these bases in real time when they interact with sunlight.
And what did you find?
Markus Oppel: The two bases absorb the light’s energy and can convert it into heat. They start vibrating, release the heat, and remain undamaged.
Is that a new discovery or something already known?
Markus Oppel: There were already static clues as to how it might work – but we were the first to simulate what actually happens in real time. That’s one of our strengths: we can show how a molecule interacts with light, including its surroundings. In the case of DNA bases, that’s typically an aqueous solution, as in human tissue. So we can simulate the molecule together with its environment and see the bigger picture rather than just the isolated part. That gives us much clearer insight into these processes.
“
There had already been static indications of how this might work – but for the first time, we were able to simulate what actually happens in real time.
„

Especially in this DNA project, we were able to uncover why transplant patients face an increased risk of skin cancer due to certain medications. We identified the mechanism behind a well-known phenomenon. One of these base pairs is guanine–cytosine. You can replace an oxygen atom in guanine with a sulphur atom – something that’s actually done in practice. The result is a class of compounds used as immunosuppressants for transplant patients. It’s known that these modified amino acids increase the risk of skin cancer. We were able to show that the sulphur makes the bases less resistant to sunlight: instead of converting light into harmless heat, the energy remains trapped and damages the cell.
Looking to the future: is there a scientific breakthrough that would make you say, “All that work on HPC systems was worth it”?
Markus Oppel: I’d say we’re far too down to earth for that. Science today is a multiplayer game. We work in teams, and many teams work on the same problems. It’s all about lots of small pieces of a puzzle. When the big picture is finally there, framed and hanging on the wall, the question isn’t who painted it – because everyone contributed.
So scientists need to be real team players?
Markus Oppel: Absolutely. I tell that to first-year students as well: work together. From the very start, I encourage them – when you’re solving problems, collaborate. Even if it means one person does more and the others copy: what matters is that people sit together and use each other’s strengths for the good of the group.
“
It’s important for people to sit down together and make use of each individual’s strengths for the benefit of the group.
„
About
Dr Markus Oppel is a Senior Scientist at the Institute of Theoretical Chemistry at the University of Vienna, and an expert in high-performance computing (HPC) and quantum computing in chemistry. His work includes managing the institute’s in-house supercomputing cluster, advising researchers on the optimal use of large-scale computing resources, and supporting research projects on European high-performance systems. He also teaches courses such as mathematics for chemists and high-performance computing in chemistry. Oppel’s research focuses on the simulation of chemical processes, quantum dynamics, and the use of quantum computers in drug and materials research.
